

However, when two elements combine in a compound, it is insufficient to merely determine the percentage of each element present to obtain the correct atomic weight. Values given in the table indicate that Dalton grasped the ideas of constant composition in compounds and of multiple proportions. (It might be noted that the Manchester Society was England's oldest continuing scientific society, other than the Royal Society of London). In an attempt to explain the problem of the variation of gaseous solubilities discovered by William Henry and its effect on his own theory of mixed gases, Dalton compared these relative weights of atoms.4 The originality of Dalton's work was his calculating system to compare the weights of chemical particles.5 The first table of atomic weight values was given by him in a paper entitled, "On the Absorption of Gases by Water and Other Liquids." It was read to the Manchester Literary and Philosophical Society, of which Dalton was secretary, on 21 October 1803. Since it had the smallest atomic weight value, Dalton chose hydrogen as his reference scale unit, hydrogen = 1 and he calculated atomic weights by comparing weights of other atoms with that of hydrogen. It is possible to assign relative weights by determining the ratio in elements reacted with each other. He assigned weights to atoms and expressed the relations between atoms of elements in precise numerical terms. An inquiry into the relative weights of these ultimate particles of matter was an entirely new subject with Dalton. The English school teacher, John Dalton, is usually given credit for the first clear statement of atomic theory and for proceeding to establish it on a thoroughly sound experimental basis, when he tested Proust's law and noted that the same elements combined in different proportions to produce different substances. However, Greek atomism had taken on the connotation of atheism to the English clergy.2 Newton rarely used the term 'atom', although his work was rooted in an atomistic theory of matter.3
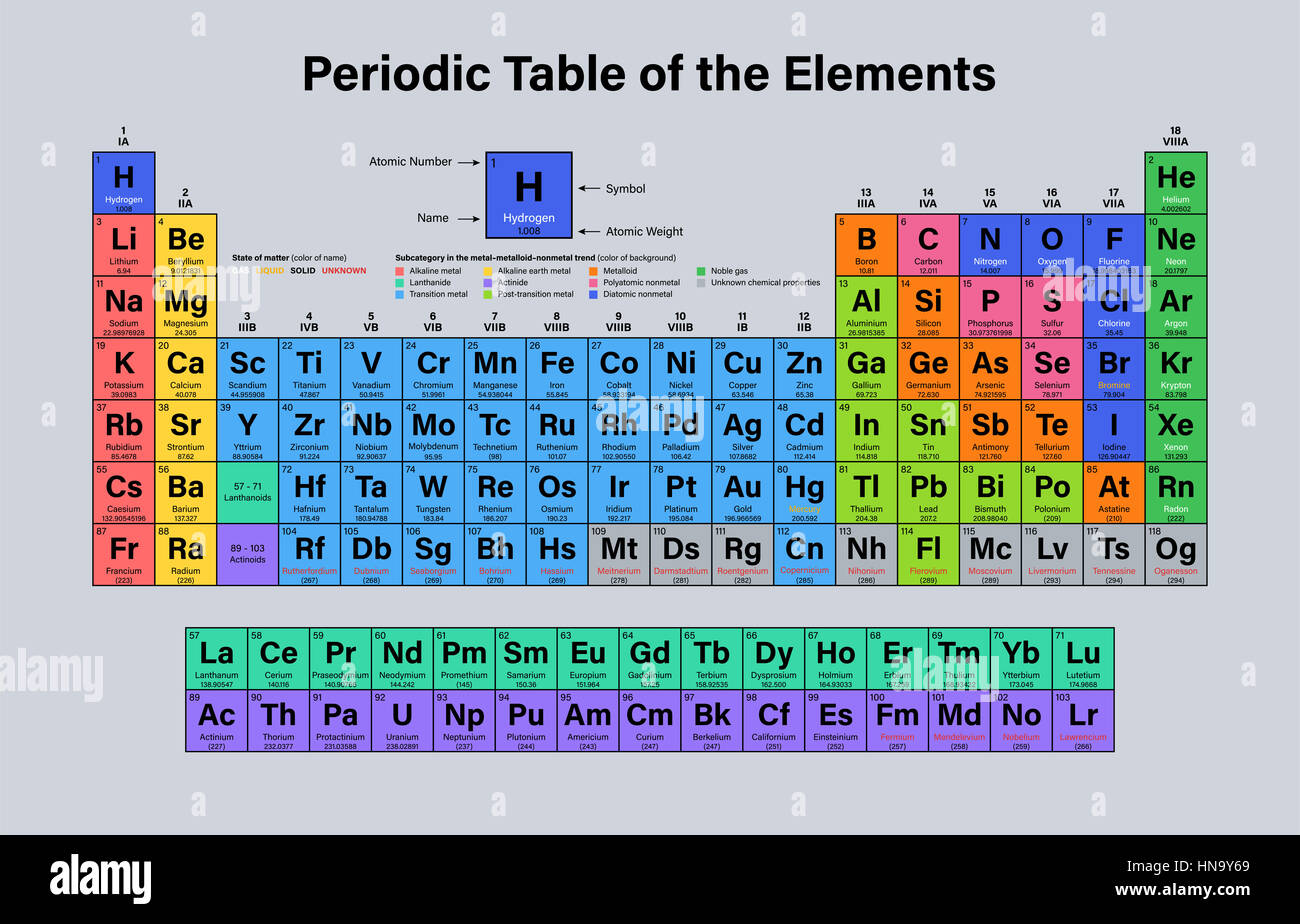
The French chemist Joseph Proust's analyses showed that a particular chemical compound always contains the same elements combined in the same mass ratio. He determined that a given amount of matter has a total mass, which remains the same when it changes in chemical combination, whether in the solid, liquid or gaseous state. In the late eighteenth century, the French scientist Antoine Lavoisier revolutionized chemistry by the introduction of accurate weighing. This is the definition that is still in use today. He defined an element as a material that could be identified by scientific experiment and could not be broken down into still simpler substances. He developed chemical analysis-the technique for breaking down substances into their most elementary parts. The 16th century alchemist Paracelsus added to those elements his three principles in matter, i.e., sulfur, salt and mercury.īy the seventeenth century, the Irishman Robert Boyle denied that these seven were the basic elements. They taught that all matter was composed of four elements: fire, water, air and earth. In the Ionian culture of Greece 2500 years ago, Leukippos, Demokritos and Epikuros philosophized that all matter was composed of atoms1 (Greek: indivisible). Speculation on the ultimate form of matter has engaged philosophers from the earliest times. Holden, when chairman of the commission from 1979-1983.


 0 kommentar(er)
0 kommentar(er)
